Abstract
Isocitrate dehydrogenase (mIDH)1/2 mutations are present in ~20% of Acute Myeloid Leukaemia (AML) and result in neomorphic IDH enzymes that produce the oncometabolite d-2-hydroxyglutarate (2HG) and differentiation arrest. MIDH inhibitors including Enasidenib (ENA) and Ivosidenib, which inhibit mIDH2 and mIDH1 respectively, have been approved for treatment of relapse-refractory AML. However, their efficacy as monotherapies is limited. AG221-AML-005 is a randomised, multi-centre phase 1b/2 clinical trial to establish the efficacy of the combination of ENA+AZA (ENA/AZA), compared to AZA alone, in patients with newly diagnosed mIDH2 AML (NCT02677922, Dinardo et al. 2021), and we aimed to uncover molecular mechanisms of response and therapy resistance in this cohort.
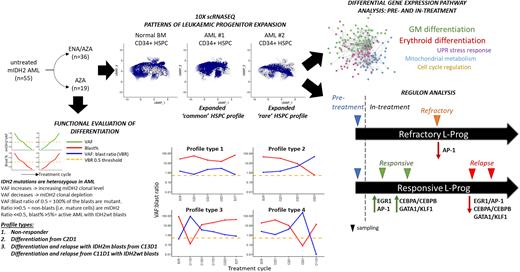
We analysed 55 patients from the AML005 cohort. 80% of patients had R140Q and 20% had R172 codon mutations. 19 were treated with AZA alone and 36 with combination. 96% of patients had a CD34+ blast immunophenotype, consistent with either a lymphoid-primed multipotent progenitor /granulocyte-macrophage progenitor-like (76%) or multipotent progenitor /common myeloid progenitor-like (20%) leukaemic progenitor (L-Prog) and differentiation arrest. Combining FACS analysis and IDH2 genotyping of longitudinal bone marrow (BM), we calculated variant allele frequency (VAF):blast ratios to track patient and to estimate mIDH2 clonal burden and differentiation. We were able to discriminate bona fide differentiation (mature mIDH2 blood cells) from clono-toxic (depletion of mIDH2 stem cell) phases of therapy. In responsive patients, we observed distinct VAF:blast profiles that show that ENA/AZA stimulates differentiation within the initial 3 to 5 cycles of treatment, and that it does more frequently than AZA alone (73% vs. 27%). We observed multiple genetic mechanisms of relapse to ENA/AZA in this cohort, including acquisition or expansion of IDH1 mutant clones, or emergence of mutations in GATA2, FLT3 and TET2, with or without persistence of the IDH2 mutant clone.
We used single cell CITE-seq (Stoeckius et al. 2017) in pre-treatment, on-treatment and relapsed samples in responsive and non-responsive patients in both treatment arms to identify transcriptional networks associated with response or resistance. Independent of their flow cytometric immunophenotypes, we found are two broad transcriptomic L-Prog profiles in mIDH2 AML compared with normal BM: expanded progenitor subpopulations which are i) rare (<8% of CD34+) or ii) common (>92% of CD34+) in normal BM. Furthermore, all studied patients had at least 2 distinct L-Prog populations, highlighting intra-tumoural transcriptional heterogeneity.
During early response to treatment, cellular profiles became more similar to normal BM with marked increase in differentiated populations particularly in ENA/AZA treated patients. By comparing paired mIDH2 L-Prog populations pre- and early on-treatment ENA/AZA and AZA samples (within 5 cycles), we studied critical changes to gene networks and pathways occurring in prior to depletion of the dominant mIDH2 clone. In ENA/AZA-treated patients, core pathways of GM and erythroid differentiation were upregulated in all studied responsive L-Prog. However, different patients employed different additional pathways, including those involved in cell cycle regulation, mitochondrial metabolism and the unfolded protein stress response. Examination of differences between patients responding to ENA/AZA versus AZA alone may provide mechanistic insights that help explain superior response rates of combination therapy.
We performed SCENIC (Aibar et al. 2017) analysis to identify gene regulatory networks (Regulons) comparing L-Prog populations longitudinally pre and on-treatment, in responding, primary refractory and relapse samples in ENA/AZA-treated patients. Response was associated with initial expression of EGR1 and JUN/FOS (AP-1) regulons, followed by CEBPA/B with GATA1/ KLF1 driving GM and erythroid differentiation respectively. Crucially, all three networks were downregulated in L-Prog from relapse in the same patients, and AP-1 regulons were also downregulated in a refractory patient. By studying early on-treatment samples, we discovered gene networks are critical for the priming of different L-Prog subpopulations by ENA/AZA therapy to subsequently differentiate into mature GM or erythroid cells.
Disclosures
Vyas:Daiichi Sankyo: Honoraria; Astellas: Honoraria; Abbvie: Honoraria; Pfizer: Honoraria; Bristol Myers Squibb: Research Funding; Celgene: Honoraria, Research Funding; JAZZ: Honoraria. Gandhi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Risueño:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties. Hasan:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal